Patients with COVID-19 and underlying hematological malignancy seem to have a higher mortality rate compared with those patients without malignancy, however, the extent of such excess risk is unclear. We performed a systematic review of literature and a pooled analysis to provide precise estimates of the mortality rate among patients with both hematological malignancy and COVID-19.
Methods: We performed a systematic literature search including peer-reviewed publications, preprints, and conference proceedings up to July 16, 2020. Only studies including exclusively patients with hematological malignancies were considered. The primary endpoint was the case fatality rate (CFR), which was defined as rate of death in patients with hematological malignancy and COVID-19. A random effects model was used to derive a pooled CFR and its 95% confidence interval (CI).
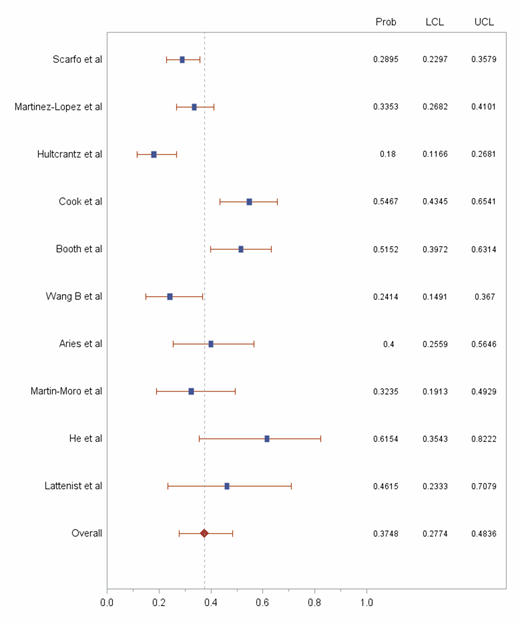
Results: In total, 10 studies including 751 patients with both COVID-19 and hematological malignancy were selected for the pooled analysis (Table 1). A total of 257 deaths were recorded in this population. The probability of death was 37·48% (95% CI 27·74% to 48.36%; I2=48·1%) in this patient population (Figure 1). [Data will be updated closer to the ASH 2020 meeting].
Conclusions: Patients with both COVID-19 and hematological malignancy have a higher probability of mortality compared to patients with COVID-19 but without underlying malignancy. Optimally and tailored preventive measures are needed to reduce the risk of COVID-19 infection in patients with hematological malignancies; this patient population should be priority for vaccine campaigns.
Saini:Covance Inc.: Current Employment; European Commission: Other: Consulting fees. Tagliamento:Roche: Other: Travel grant; Bristol-Myers Squibb: Other: Travel grant; AstraZeneca: Other: Travel grant; Takeda: Other: Travel Grant; Novartis: Honoraria; Amgen: Honoraria. Lambertini:Roche: Other: Consultant; Novartis: Other: Consultant; Roche: Speakers Bureau; Takeda: Speakers Bureau; Lilly: Speakers Bureau; Novartis: Speakers Bureau; Pfizer: Speakers Bureau; Theramex: Speakers Bureau. McNally:Covance Inc.: Current Employment. Kelly:Covance Inc.: Current Employment. Romano:Covance Inc.: Current Employment. Chico:Covance Inc.: Current Employment. Jenson:Laboratory Corporation of America: Current Employment. Anderson:Laboratory Corporation of America: Current Employment, Current equity holder in publicly-traded company; OmniSeq: Membership on an entity's Board of Directors or advisory committees. Curigliano:Novartis: Other: personal fees for consulting, advisory role and speakers' bureau ; Pfizer: Other: personal fees for consulting, advisory role and speakers' bureau ; Lilly: Other: personal fees for consulting, advisory role and speakers' bureau ; Foundation Medicine: Other: personal fees for consulting, advisory role and speakers' bureau ; Samsung: Other: personal fees for consulting, advisory role and speakers' bureau ; Daichii-Sankyo: Other: personal fees for consulting, advisory role and speakers' bureau ; Ellipses Pharma: Honoraria; Roche/Genentech: Other: fees for travel and accommodation ; Pfizer: Other: fees for travel and accommodation ; Roche/Genentech: Other: personal fees for consulting, advisory role and speakers' bureau . de Azambuja:Roche/Genentech: Other: honoraria and advisory board fees and travel grant, and research grant to institution; Novartis: Other: honoraria and advisory board fees and travel grant and research grant to institution; Seattle Genetics: Other: honoraria and advisory board fees ; GSK: Other: Travel grant and research grant to institution; AstraZeneca: Other: Research grant to institution.
Author notes
Asterisk with author names denotes non-ASH members.

This feature is available to Subscribers Only
Sign In or Create an Account Close Modal